Macrocyclic peptides: the next wave of drug discovery
Merck scientists say the “Goldilocks” chemical modality could lead to new ways to impact disease
September 14, 2023

Small molecules, generally taken as pills, make up nearly 90% of medicines used today. It’s hard to think of a world without them. The use of small molecules has been critical in expanding the reach of and access to medicines around the world.
But it’s challenging for small molecules to impact the large featureless surfaces of protein-protein interactions, which govern a wide range of biological processes in our bodies. To target these interactions, scientists have turned to large molecule biologic therapies, like monoclonal antibodies, which — taken by infusion or injection — have been critical in advancing the treatment of many diseases, including some cancers and autoimmune disorders.
Over a decade ago, Merck scientists began investigating a way to engineer a new type of medicine combining the ease-of-administration of a small molecule with the potency and target specificity of an antibody.
Macrocyclic peptides have shown promise in achieving this balance.
“Macrocyclic peptides allow us to cast a wider net on the protein interactions we want to drug, providing a vast and untapped opportunity to access a wider range of targets and potentially new ways to treat different diseases,” said Dani Schultz, director of chemistry, Merck Research Laboratories.
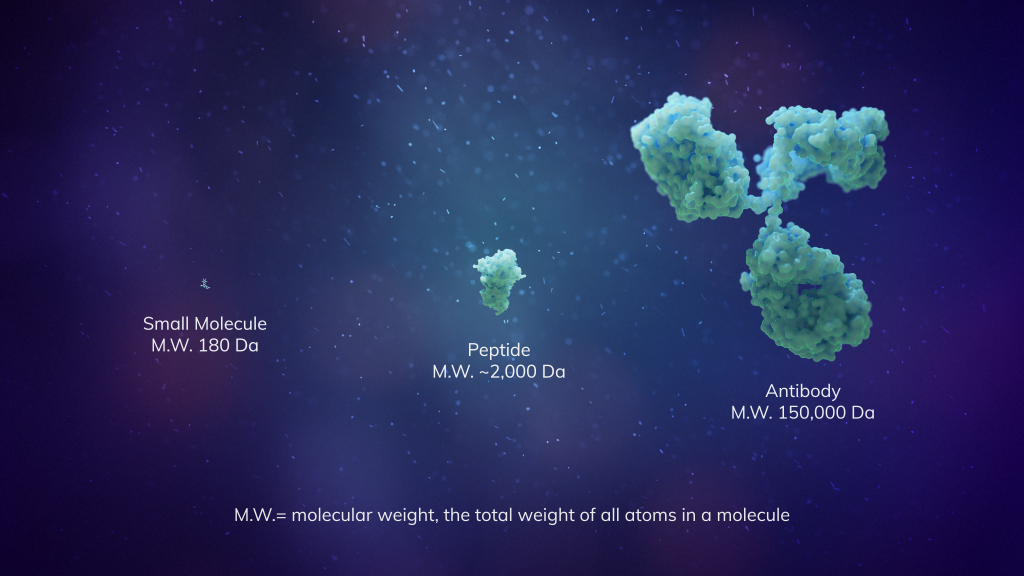
Not too big, not too small: the “Goldilocks” modality
Macrocyclic peptides have been called the “Goldilocks” chemical modality because their intermediate size combines the favorable properties of both small molecules and biologics¹. And thanks to their unique ring shape, macrocyclic peptides can cover more surface area to potentially disrupt protein-protein interactions more so than traditional, linear-shaped peptide therapies.
“The design and invention of macrocyclic peptides is notoriously complicated,” said David Thaisrivongs, director of chemistry in Merck Research Laboratories.

“Similarly, scaling production up for a macrocyclic peptide small molecule, with four to five times the size and complexity of a typical small molecule, represented a bold endeavor.”
- David Thaisrivongs
Director of chemistry, Merck Research Laboratories
For Merck, this work started by screening large libraries of cyclic peptides using messenger RNA display technology. This led to the identification of cyclic peptide leads that were optimized using 3-dimensional protein structure-based design and advanced computational techniques. Further molecular iterations and refinements improved the absorption, potency, and stability of the first candidate.
“A diverse, interdisciplinary team of skilled and determined people from across our chemistry organization has dedicated substantial efforts to advancing this science,” said Thaisrivongs.
A peptide renaissance
These macrocyclic peptide discovery efforts may one day allow us to treat diseases that have long evaded traditional small molecule approaches or improve access to medicines previously available only as an injectable.
“Macrocyclic peptides are a new modality and we’re still in the early stages of understanding their potential to impact disease and patient care,” said Schultz.
“There’s no playbook here, we’re innovating and developing new techniques on how to optimize and synthesize macrocyclic peptides — it’s really thrilling for me as a scientist because the potential is huge.”
- Dani Schultz
Director of chemistry, Merck Research Laboratories

[1] Beyond 20 in the 21st century: strengths, opportunities, and challenges of non-canonical amino acids in peptide drug discovery. Jennifer L. Hickey; A Dan Sindhikara; B Susan L. Zultanski and Danielle M. Schultz


